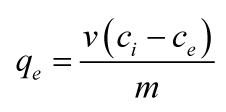|
|
Original Article
| ||||||
| The kinetics of eosin yellow removal from aqueous solution using pineapple peels | ||||||
| Fabian Audu Ugbe1, Victoria Abiola Ikudayisi1 | ||||||
|
Lecturer, Department of Science Laboratory Technology, Benue State Polytechnic, Ugbokolo, Benue State, Nigeria
| ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
[Print This Article]
[Similar article in Pumed] [Similar article in Google Scholar] |
| How to cite this article |
| Ugbe FA, Ikudayisi VA. The kinetics of eosin yellow removal from aqueous solution using pineapple peels. Edorium J Waste Manag 2017;2:5–11. |
|
ABSTRACT
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Color impurity in industrial effluents constitutes a significant risk to human health and the environment, so much effort has been expended to degrade them using various methods, including the use of agricultural waste materials as adsorbent. The purpose of this study was to provide understanding of the mechanisms for the removal of eosin yellow from aqueous solutions onto pineapple peels as adsorbent. The adsorption equilibrium was studied on effects of initial adsorbate concentration, initial solution pH, and contact time using batch equilibrium techniques. The fitness of equilibrium data to common kinetic models such as pseudo first order, pseudo second order, intraparticle diffusion and film diffusion were tested. From the results obtained for the adsorption experiments, the best adsorption potential was recorded at eosin yellow concentration of 250 mg/L (qe =12.49 mg/g), agitation time of 90 minutes (qe=13.35 mg/g) and initial solution pH of 3 (qe= 22.01 mg/g). Results of kinetic study revealed the order of fittings: Intraparticle diffusion (R2 = 0.981) > Pseudo first order (R2 = 0.978) > Liquid film diffusion (R2 = 0.972) > Pseudo second order (R2 = 0.96). The goodness of fit was observed with the various kinetic models tested, indicating the applicability of these models in the adsorption of eosin yellow on pineapple peels. Thus, the results of the study could provide useful information to evaluate pineapple peels for the practical removal of eosin yellow from aqueous solution. Keywords:Adsorption, Eosin yellow, Kinetic models, Pineapple peels | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
INTRODUCTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Water is a basic necessity for life on earth. About 2.66% of the total global water resources (groundwater, lakes and rivers, polar ice and glaciers) are fresh water, but only a small fraction (0.6%) is available as drinking water [1]. Water pollution is the contamination of water bodies such as lakes, rivers, oceans, and groundwater caused by human activities, which can be harmful to organisms and plants which live in these water bodies [2]. Dyes are being used by a large number of industries for diverse purposes such as dyeing, printing, cosmetics, pharmaceuticals, leather, printing ink and fluorescent pigment. It is also used in special culture media. These are done in order to color their products and make their appearance more attractive. Textile dyeing industry uses a lot of synthetic dyes due to their superior dyeing properties, especially in terms of fastness. This industry emits significant amounts of synthetic textile organic dye wastes amongst all industrial waste waters [3]. Therefore, it is necessary that the water reserves be treated carefully and wastewater treatment be done [4]. Adsorption appears to be good for the treatment of effluents. The first thing for an efficient adsorption process is the search for a low cost adsorbent with high adsorption capacity and secondly, its biodegradability [5]. Eosin Y is a pink water soluble acid dye (anionic dye) which also displays yellow-green fluorescence and with the wavelength of maximum absorbance (λmax) of 517 nm [6]. The effective treatment of the Eosin Y effluent is eco-friendly to aqueous environment, otherwise, it can lead to severe health problems due to its carcinogenic properties [7]. The chemical structure of eosin yellow is shown in Figure 1 [6]. Pineapple (Ananas comosus) holds the third rank in the world tropical fruit production after banana and citrus. Pineapple is the edible member of the family Bromeliaceae [8]. Few researchers have investigated the removal from aqueous systems of Eosin Y by different adsorbents such as modified sawdust [7], conditioned chitosan hydrobeads [6] and EDTA modified chitosan [9]. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
MATERIALS AND METHODS
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Adsorbent and adsorbate preparation | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
The pineapple peels was collected from pineapple traders along market road at Ugbokolo, Edumoga in Benue State, Nigeria. A sample of about 2 kg (estimated to be enough for the entire sorption process) was collected and air-dried in the laboratory at room temperature for five days. The sample was then pretreated according to the method reported by [10]. The sample was then passed through a 0.112 mm mesh sieve and used for subsequent experiment. The adsorbate, Eosin Y was purchased from Sigma Aldrich (Germany) and was used for the adsorption experiments without further purification. A 1 gram of Eosin Y was weighed and dissolved in 1000 ml of deionized water to make 1000 ppm, from which lower concentrations were prepared by serial dilution method.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Adsorption experiment | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
A 0.1-g portion of the pineapple peels sample was weighed into different 100 mL conical flasks and 15 cm3 of working standard solution (25, 50, 75, 100, 150, 200, 250 and 300) ppm of the Eosin Y dye was added separately to the pineapple peel sample in the flask. Each solution was agitated on a flat orbital mechanical shaker for four hours and then filtered. The filtrate was analyzed using ultraviolet-visible spectrophotometer at 517 nm to determine the quantity of Eosin Y remaining in the solution [11]. The equilibrium concentration (concentration with the highest adsorption capacity) was then selected for use in subsequent experiments. Further experiments were carried out using the equilibrium concentration to examine the effects of initial solution pH (varied from 2–8) and agitation time (varied from 5–160 minutes). The amount of the dye adsorbed by the adsorbents at equilibrium was determined using a mass balance equation [11]: Where qe is the quantity adsorbed by the adsorbent (mg/g), V is the volume of the adsorbate used (L), m is the mass of the adsorbent (g), ci and ce are the initial and final concentrations of the adsorbate (mg/L) respectively. The kinetic study is necessary for an adsorption process because it depicts the uptake rate of the adsorbate towards the adsorbent and controls the remaining time of the whole adsorption process [12]. The kinetic study has the important practical task to determine the degree of utilization of the adsorption capacity as a function of the time of contact between the liquid and the solid in adsorption process. In the present study, four models were being treated. Pseudo first order is the earliest model used to describe adsorption rate pertaining to adsorption capacity [13] with the linear equation stated below [14]: Where qe and qt (mg/g) are the adsorption capacities at equilibrium and time t (min) respectively, K1 (min-1) is the pseudo first order rate constant. The values of K1 and qe can be obtained from the slope and intercept respectively of the linear plot of ln (qe–qt) against t. On the other hand, pseudo second order model is based on the assumption that the rate-limiting step may be chemical sorption (chemisorption) involving valence forces through sharing or exchange of electrons between the adsorbent and the adsorbate. In addition, it is assumed that the sorption capacity is proportional to the number of active sites occupied on the adsorbent [15][16] with the equation stated below: Where qe and qt (mg/g) are the adsorption capacities at equilibrium and time t (min) respectively, and K2 (g/mg/min) is the pseudo second order rate constant. The values of qe and K2 can be obtained from the slope and intercept respectively of the linear plot of t/qt against t. There are several steps involved in the adsorption of adsorbate by an adsorbent. These steps (which can be the rate controlling steps) involve transport of the solute molecules from the aqueous phase to the surface of the solid particulates (film diffusion) and diffusion of the solute molecules into the interior of the pores (intraparticle diffusion), which is usually a slow process, these steps are being referred to as the mechanism of adsorption and cannot be explained by the kinetics of chemical reactions [17][18]. In order to establish the rate controlling step of the uptake of Eosin Y dye on pineapple peels, intraparticle diffusion equation also known as Weber—Morris equation was used as stated below: Where Kid (mg/g/min1/2) is the intraparticle diffusion rate constant or coefficient, and can be obtained from the slope of the linear plot of qt against t1/2. C is the intercept; the boundary layer thickness is described by the values of the intercept. The larger the intercept, the greater is the boundary layer effect [17]. Film diffusion on the other hand is given by [18][19]:
Where Rl (min-1) is liquid film diffusion constant and can be obtained by the linear plot of ln [1 – qt/qe] against t. qt and qe are the quantity adsorbed at time t and at equilibrium respectively. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
RESULTS
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Effect of initial adsorbate concentration | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
The result of effect of initial eosin Y concentration on its sorption by pineapple peels is shown in Figure 2. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Effect of initial solution pH | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
The effect of initial solution pH on the adsorption of the dye on pineapple peels was studied and the result is shown in Figure 3. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| EEffect of variation of contact time | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
The effect of variation of contact time was investigated in the range of 5–160 min and the result is presented in Figure 4. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Kinetic models | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Four models namely, pseudo first order, pseudo second order, intraparticle diffusion and film diffusion models were being used to fit the obtained kinetic curves in order to define the rate parameters and explain the mechanism of mass transfer.
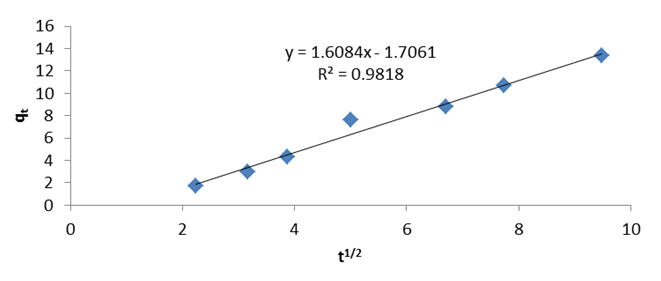
The parameters obtained from the models are presented in Table 1 and Table 2. The plots of data obtained from the intraparticle diffusion and film diffusion studies are presented in Figure 5. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
|
DISCUSSION
|
| Effect of initial adsorbate concentration
It was observed from Figure 2 that the percentage adsorption of the pineapple peels for Eosin Y decreased considerably with increase in the initial concentration due to the saturation of the active sites on the adsorbent surfaces at increased concentration while the quantity adsorbed per gram (qe) increased due to the high availability of adsorbate ions in solution at increased concentration [7]. The maximum quantity adsorbed, qe of 12.49 mg/g was obtained at 250 ppm concentration of the adsorbate (eosin yellow). |
Effect of initial solution pH
|
| The initial pH of a solution is also a very important factor to be considered in adsorption studies as it has been observed to play a major role in the adsorption of different adsorbates by various adsorbents. This is because it dictates the degree of ionization of the adsorbates and the surface properties of the adsorbents which in turn, will affect the rate of adsorption [20]. It has been reported that eosin yellow precipitates at pH lower than 2 [7], so the pH studied ranged from 2–8. It is shown in (Figure 3) that the adsorption of eosin yellow (EY) on pineapple peels was maximum at pH of 3 with quantity adsorbed being 22.01 mg/g. This observation of maximum adsorption capacity at lower pH may be attributed to the electrostatic interaction between the negative charge of the dye and the protonated groups on the surface of the adsorbents at lower pH (acidic conditions) due to the presence of H+ ions, and low adsorption capacity at higher pH may be as a result of the presence of OH- groups which compete with the negative charge of the dye for the active sites on the surface of the adsorbents. Similar result was reported by [6].
Effect of variation of contact time
|
| Contact time effect has a greater influence on adsorption process. The effect of time helps to know the rate of reaction and the time the adsorption process reaches equilibrium [21]. It was observed from Figure 4 that there was gradual increase in the adsorption capacity as the time increases due to the active sites being progressively filled with time until it reached equilibrium at 90 minutes after which the adsorption maintained a fair constancy. The quantity adsorbed increased from 1.70 mg/g–13.35 mg/g as time increased from 5 min–90 min, with no possibility of increase in quantity adsorbed after 90 min as can be seen from the nature of the curve. Similar result was reported elsewhere by Abdus-Salam et al. [22].
KINETICS STUDY
|
| Pseudo first order and pseudo second order kinetics
Table 1 showed that both pseudo first order and pseudo second order showed good fit to the experimental data by having their correlation coefficients close to unity. However, from the values of their correlation coefficients (R2), pseudo first order (R2 = 0.978) showed a slightly better fit than the pseudo second order kinetics (R2 = 0.96). Also, the theoretically determined qe (11.76 mg/g) for pseudo first order model is fairly close to the values obtained experimentally (13.35 mg/g) [23]. The good applicability of the pseudo first order model in the sorption process indicates that one eosin Y ion is sorbed onto one sorption site on the adsorbent surface as also reported elsewhere by Boparai et al. [23].
|
Intraparticle diffusion model and film diffusion models
|
| It was shown from the plots (Figure 5) and Table 2 that the two processes, intraparticle diffusion (R2 = 0.981) and film diffusion (R2 = 0.972) contribute to the mechanism of the adsorption with their R2 values found to be very close to unity. Also, their plots did not pass through the origin which showed that none of the two processes is the sole rate limiting step or that other processes occurred simultaneously with these processes in the mechanism of the adsorption [12]. The intraparticle curve which showed higher R2 value depicts that the slowest step in the mechanism of the adsorption is diffusion into the internal pores of the adsorbent, thus, intraparticle diffusion dominates film diffusion in the mechanism of the sorption. Similar result was obtained by Ajaykumar et al. [24].
CONCLUSION
|
| From the adsorption data, the sorptive property of the pineapple peels was found to be dependent on initial adsorbate concentration, initial solution pH and contact time. Adsorption data was also found to fit both the pseudo first and pseudo second order kinetic models. The mechanism of the adsorption process investigated showed that intraparticle diffusion is not the sole rate controlling step. Therefore, the results of the study could provide good information to evaluate pineapple peels for the practical removal of Eosin Y from wastewater.
REFERENCES
|
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||